Abstract
Introduction Myelodysplastic syndrome (MDS) is a diverse group of clonal myeloid neoplasms. The accumulation of mutations in stem cell compartments is involved in MDS pathogenesis. Mounting evidences demonstrate that the addition of mutation data improves the prognostic stratification in MDS patients. In addition to mutational profiles, the variant allele frequency (VAF) of individual mutations also influence the prognosis in MDS patients.
Materials and Methods A total of 698 primary MDS patients were recruited. The diagnoses were based on the 2016 World Health Organization classification. Patients with antecedent chemotherapy/radiotherapy or hematologic malignancies were excluded. TruSight myeloid sequencing panel (Illumina, San Diego, CS, USA) and the HiSeq platform (Illumina, San Diego, CA, USA) were adopted to analyze the gene alterations and mutant allele burdens. Because of the sequencing sensitivity issue, we verified CEBPA mutations and FLT3-ITD via Sanger sequencing.
Results Exploration of the correlation between prognosis and VAF of individual genes showed that compared with wild-type genes, high VAF of mutations in 9 genes, including DNMT3A (cutoff value 40%, hazard ratio HR 1.67, p<0.001), TET2 (45%, HR 1.60, p<0.001), ASXL1 (20%, HR 1.48, p<0.001), E.ZH2 (40%, HR 1.45, p=0.037), SETBP1 (15%, HR 1.39, p=0.025), BCOR (80%, HR 1.57, p=0.045), SRSF2 (50%, HR 1.89, p=0.002), ZRSR2 (60%, HR 1.70, p<0.001) and TP53 (25%, HR 2.79, p<0.001) were significantly associated with shorter leukemia-free survival (LFS). With the exception of EZH2, SETBP1, and BCOR mutations, high VAF of all other six mutations were also associated with shorter overall survival (OS). In addition, the patients with SRSF2 and TP53 mutations, even at lower VAF, had poorer prognosis for both LFS and OS compared with those with wild-type genes. For other 7 above-mentioned mutations, only higher VAF conferred poorer outcomes; the patients with lower mutation VAF had outcomes similar to the patients with wild-type genes.
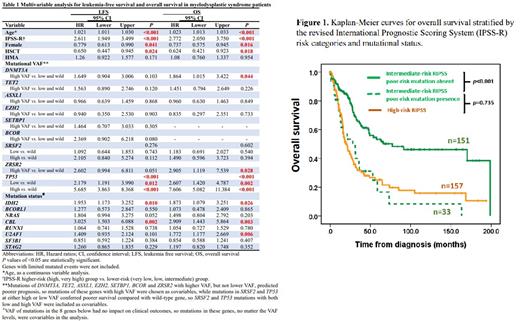
In multivariable analysis (Table 1), female sex and receiving allogenic hematopoietic stem cell transplantation (HSCT) were independent favorable factors for both LFS and OS, while older age and higher-risk IPSS-R predicted shorter LFS and OS. Mutant IDH2 and CBL predicted both shorter LFS and OS, while U2AF1 mutation and DNMT3A and ZRSR2 mutations with high VAF were associated poorer OS. The mutation status and VAF levels of TP53 mutations could distinguish patients into three hierarchical groups with different prognoses. The presence of poor-risk mutations as identified in the multivariable Cox analysis could re-stratify the IPSS-R risk groups. Patients with these unfavorable mutations in each IPSS-R risk subgroup had survivals worse than other patients of the same risk but similar to or even worse than those in the next higher-risk subgroup (Figure 1). Considering OS, the incorporation of the molecular data in the IPSS-R could reclassify 8.9% (18/202) of IPSS-R very low/low-risk patients to intermediate-risk subgroup, 17.9% (33/184) of IPSS-R intermediate to high-risk subgroup, and 34.4% (54/157) of IPSS-R high to very high-risk subgroup. Patients harboring U2AF1 mutation had poorer LFS and OS if they were not treated with hypomethylating agents (HMA), but had similar LFS (wild-type vs. mutant-type, 14.3 vs. 15.5 months, p=0.537) and OS (wild-type vs. mutant-type, 22.2 vs. 40.1 months, p=0.450) compared with those with wild-type U2AF1 if they received HMA treatment. Moreover, patients with low TP53 mutant VAF got benefit from HMA treatment considering LFS.
Conclusion We provide evidences that VAF is critical for risk stratification in MDS patients and should be considered in novel scoring systems. Our data fostered our understanding of the mutation burden of the diseases and provided future patient-tailored therapeutic avenues.
Disclosures
Chou:aether AI: Other: The AI product in this abstract... investigators of this study w/commercial profit; National Taiwan University Hospital: Other: The AI product in this abstract... investigators of this study w/commercial profit. Hou:AbbVie, Celgene, Kirin: Research Funding; AbbVie, Astellas, AstraZeneca, BMS, Chugai, CSL Behring, Daiichi Sankyo, IQVIA, Johnson & Johnson, Kirin, Lotus, Merck Sharp & Dohme, Novartis, Ono, Panco Healthcare Co, Pfizer, PharmaEssential, Roche, Synmosa, Takeda, TSH Biopharm, TTY Biopharm Company, : Consultancy, Honoraria, Other: Travel.
Author notes
Asterisk with author names denotes non-ASH members.